Abstract
Systematic review of infectious events in prospective trials of Ibrutinib
Ibrutinib is an oral small molecule inhibitor of Bruton tyrosine kinase (BTK). It is efficacious in the treatment of multiple B cell lineage disorders including chronic lymphocytic leukemia, mantle cell lymphoma and Waldenström macroglobulinemia. Use is expanding with ongoing clinical trials in solid tumor oncology as well as recent FDA approval for the treatment of chronic graft versus host disease (GVHD). As experience with newer targeted agents increases we are developing better appreciation and recognition of sometimes unanticipated toxicities. While the most common side effects with ibrutinib are rash and diarrhea, signals of concern have been seen with adverse effects related to hemorrhage, pneumonitis and recent attention to cardiac arrhythmias. Based on our own institutional experience, we updated a preliminary systematic review of infectious events in prospective trials of ibrutinib in the treatment of hematologic disorders.
We reviewed the published literature and major conference abstracts using PubMed, Google Scholar and HemOnc.org. Publications through July 1, 2017 were included in the evaluation. All prospective trials of ibrutinib for the treatment of hematologic malignancies were included in the analysis. Those trials involving solid oncologic tumors, GVHD or following allogeneic stem cell transplant were excluded. Infectious events included pneumonia, sepsis, genitourinary and respiratory tract infections, sinusitis, cellulitis, infectious colitis or febrile neutropenia. Neutropenia without fever as well as inflammatory conditions (e.g., pneumonitis) without mention of infection were not included. Pneumonia was separately annotated and included bacterial, fungal, viral or other atypical causes if identified. Events were grouped by grade according to the Common Terminology Criteria for Adverse Events, version 4.03. In multi-arm trials only the ibrutinib-containing arms were included in the analysis and we generally restricted this on an intention-to-treat basis.
From the updated literature review, 929 publications from PubMed, 772 from meeting abstracts and more than 11,000 from Google Scholar were identified. 33 peer reviewed publications and 106 abstracts reporting on 61 trials including more than 2300 patients were identified.
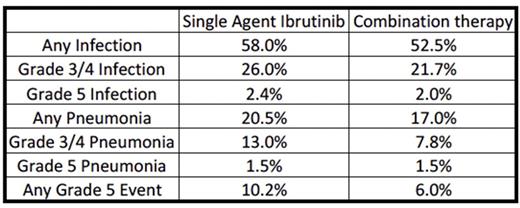
Infectious adverse events of any grade were identified in over half of the patients for which data was available. Additionally, pneumonia accounted for half of the grade 3/4 infectious events and there were 33 grade 5 pneumonias identified among the studies. A number of significant infectious events were attributed to atypical organisms such as Aspergillus spp., Cryptococcus neoformans and Pneumocystis jirovecii . Somewhat counterintuitively, the rates of all events (infections, pneumonia or any grade 5 event) were consistently lower in the studies involving combination regimens. This finding may represent a gap in the data as many of these trials are incomplete and many are only reported in abstract format at the time of this analysis. Alternatively, this may be due to a more heavily pretreated population with relatively diminished performance status enrolled in the single agent trials.
Among all studies grade 3/4 pneumonia was seen in 13% of patients on ibrutinib alone and 7.8% of patients on combination therapy. Grade 5 infections accounted for 25-33% of all grade 5 events. With increasing use of targeted therapies in patients previously not eligible for standard cytotoxic regimens, clinicians must be aware of unanticipated side effects. In one trial with a high rate of invasive aspergillosis, there was no correlation between patient CD4+ T cell counts and rates of this opportunistic infection. A separate investigation identified high rates of P. jirovecii in patients on single agent ibrutinib. Inhibition of BTK in non-malignant monocytes has been a proposed mechanism of increased susceptibility to invasive fungal disease. Antimicrobial prophylaxis with ibrutinib use is not currently standard practice in the management of hematologic diseases. Our findings of high rates of infectious events, particularly pneumonia often related to opportunistic pathogens, suggest that vigilance for infection is mandatory, and that risk factors for predisposition to infection, beyond CD4+ T cell counts and immunoglobulin levels, should be sought.
Pauff: Abbvie: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal